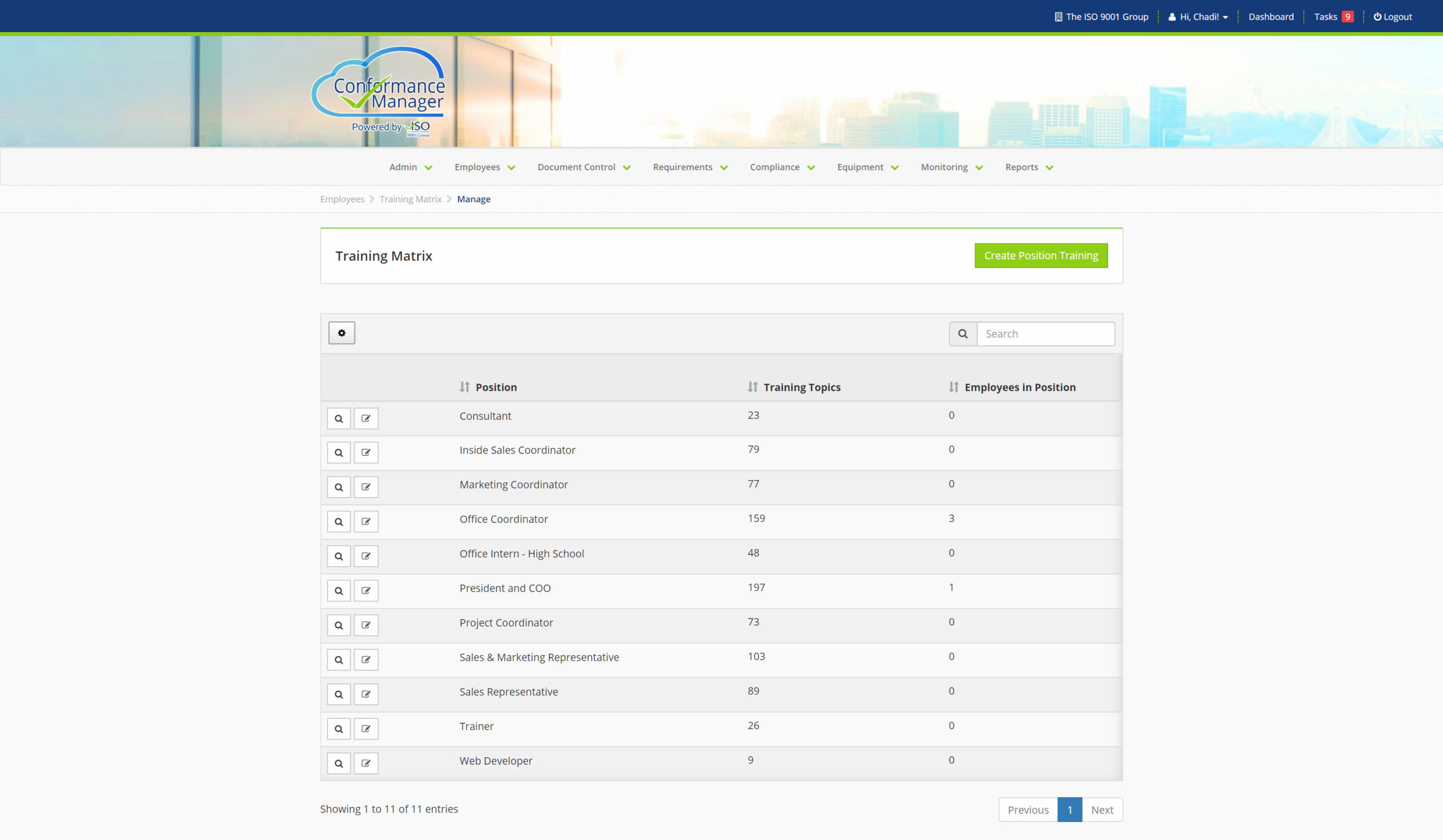
Learn About Our Modules
Nonconformity and CAPA
Capture process and product nonconformities. Associate nonconformities to controlled documents. Perform root cause analysis using the Five Why technique. Establish correction and corrective actions. Assign action(s) to users, so that they actually get done. Schedule review and follow up to verify corrective actions were effective